

I-SPY 2.2 is the latest evolution in the I-SPY family of trials for early breast cancer, introducing a number of critical innovations that permit the optimization of treatment for each patient in the context of a phase II signal-finding trial.
The success of the I-SPY 2 trial is not simply the result of a single innovation in trial design. It is the result of a pain-staking deconstruction and re-engineering of the entire clinical trial enterprise, from protocol development through registration.
Platform Design
Response Monitoring
Biological Targeting
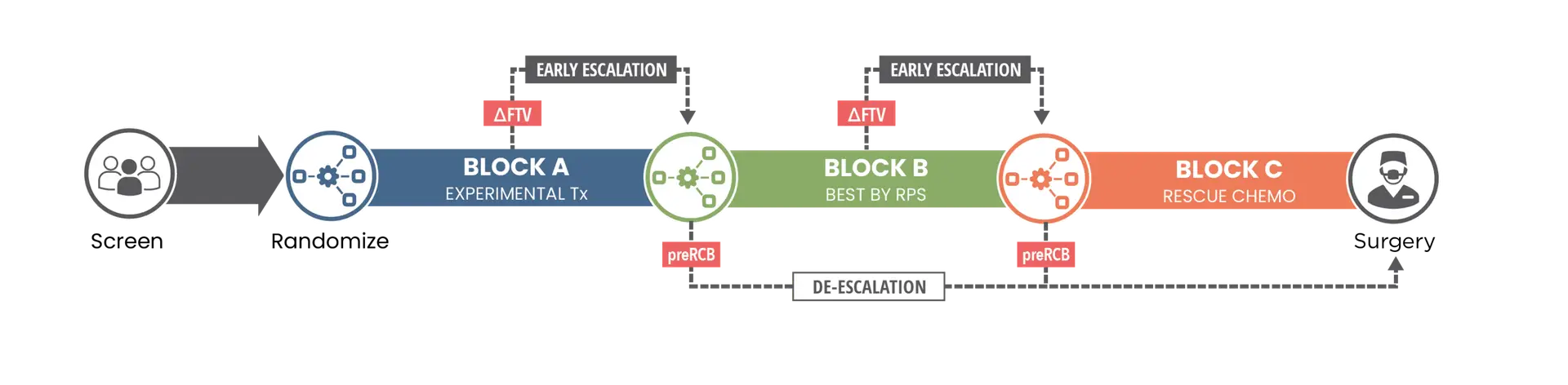
I-SPY 2.2 uses molecular ‘response-predictive subtypes’ to characterize each participant’s tumor in order to guide treatment assignments and assess subtype-specific efficacy of treatment.
Treatment Re-direction
If an experimental therapy does not work, participants are switched to an established, biologically targeted therapy. Participants may receive up to 3 ‘blocks’ of treatment in I-SPY 2.2.
Treatment Sparing
Treatment Strategies
In addition to assessing experimental agents, I-SPY 2.2 assesses whole treatment strategies consisting of multiple sequential treatments, more closely reflecting clinical approaches.
Shatsky R, Trivedi MS, Yau C, et al. Datopotamab–deruxtecan plus durvalumab in early-stage breast cancer: the sequential multiple assignment randomized I-SPY2.2 phase 2 trial. Nature Medicine https://www.nature.com/articles/s41591-024-03267-1
Khoury K, Meisel JL, Yau C, et al. Datopotamab deruxtecan in early stage breast cancer: the I-SPY2.2 sequential multiple assignment randomized phase 2 trial. Nature Medicine https://www.nature.com/articles/s41591-024-03266-2