Home › For Investigators › I-SPY ARDS/COVID Expansion
What is I-SPY ARDS/COVID Expansion Trial?
The Trial
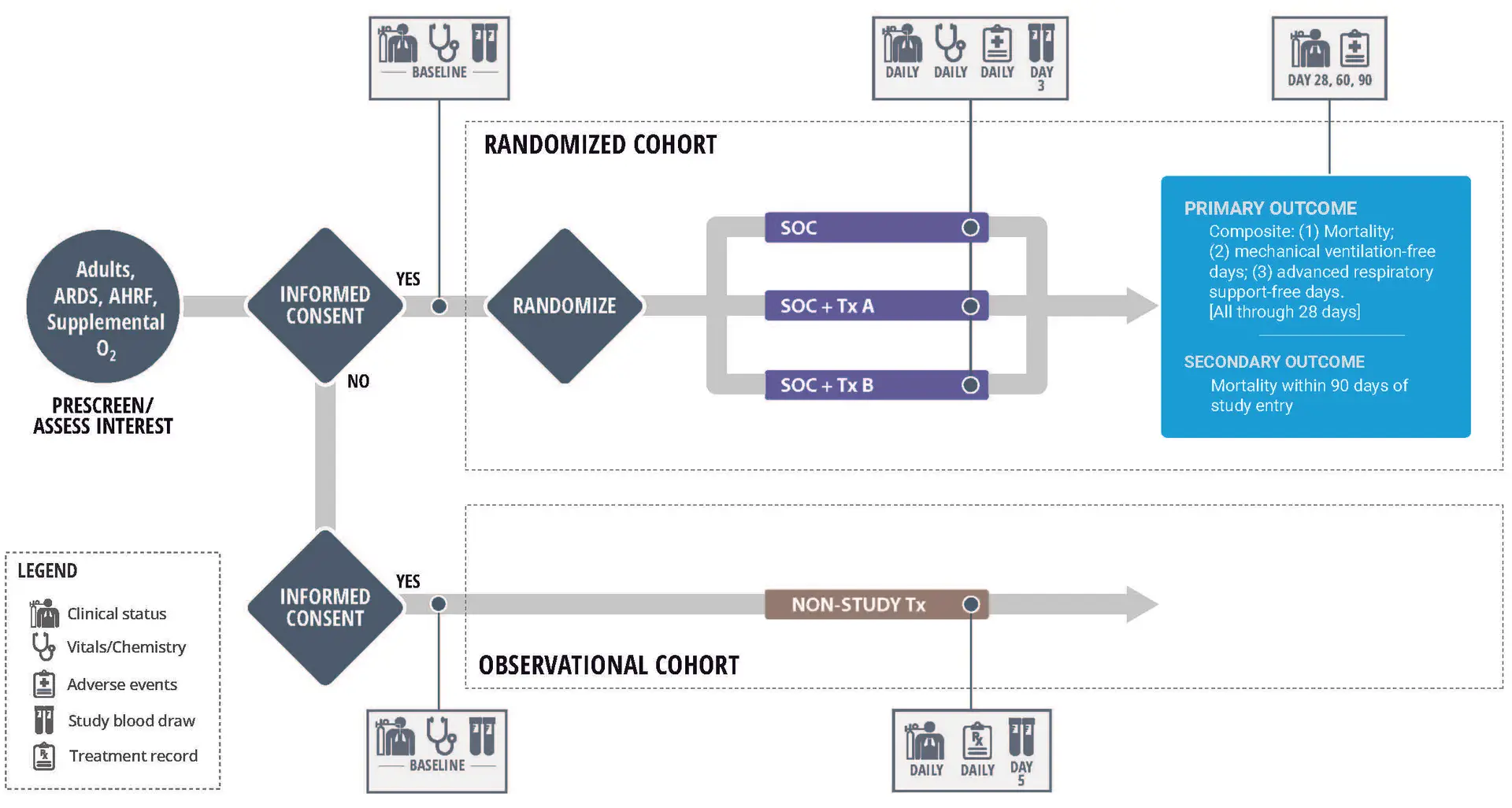
In this trial, the focus is on identifying agents that can reduce mortality and the need for, as well as the duration of, mechanical ventilation or advanced respiratory support in patients with ARDS. The trial incorporates a prespecified biomarker collection for research purposes to assess whether the data generated can lead to more targeted interventions. The goal is to quickly screen promising agents, using an adaptive platform trial design, for the treatment of critically ill ARDS and Acute Hypoxemic Respiratory Failure (AHRF) patients. The phase 2 platform design aims to identify agents with a significant impact on reducing mortality and the need for invasive mechanical ventilation. Originally focused on ARDS caused by COVID-19, the trial has transitioned to studying interventions for a broader range of causes of ARDS and AHRF.
The platform trial involves screening hospitalized patients on high-flow oxygen or intubated for eligibility. Enrolled patients, diagnosed with ARDS or AHRF, are offered participation in the therapeutic intervention phase, receiving standard care with or without an investigational agent. Those declining consent may be approached for an observational biomarker sub-study, named “Sub-Phenotypes in ARDS using Rapid Prospective Classification (SPARC: An Observational Study).” This sub-study aims to enroll participants with various causes of ARDS or AHRF and assess the ability of I-SPY medical centers to classify patients into hyper- or hypo-inflammatory sub-phenotypes in real time using biomarkers. The trial employs a randomized, controlled design with a primary endpoint focused on mortality, ventilation, and respiratory support-free days. The study also explores specific subtypes based on exploratory biomarkers, with blood samples collected for analysis. Successful agents may progress to Phase 3 trials, while an observational study concurrently collects data and biomarkers from non-participating ARDS and AHRF patients to assess subtype classification feasibility.
For more information contact Stephanie Ezrati at s.ezrati@quantumleaphealth.org
ARDS Schema
Download a List of
COVID/ARDS Trial Sites
COVID/ARDS Trial Sites
Adobe PDF (460kb)












