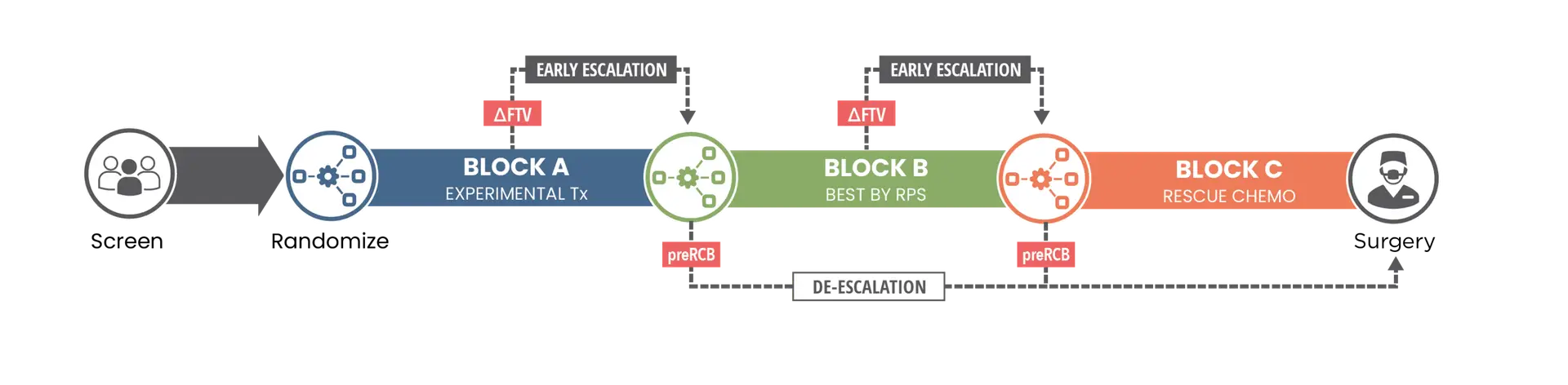
I-SPY 2.2 is the latest evolution in the I-SPY family of trials for early breast cancer, introducing a number of critical innovations that permit the optimization of treatment for each patient in the context of a phase II signal-finding trial.
The success of the I-SPY 2 trial is not simply the result of a single innovation in trial design. It is the result of a pain-staking deconstruction and re-engineering of the entire clinical trial enterprise, from protocol development through registration.
Platform Design
Response Monitoring
Biological Targeting
I-SPY 2.2 uses molecular ‘response-predictive subtypes’ to characterize each participant’s tumor in order to guide treatment assignments and assess subtype-specific efficacy of treatment.
Treatment Re-direction
If an experimental therapy does not work, participants are switched to an established, biologically targeted therapy. Participants may receive up to 3 ‘blocks’ of treatment in I-SPY 2.2.
Treatment Sparing
Treatment Strategies
In addition to assessing experimental agents, I-SPY 2.2 assesses whole treatment strategies consisting of multiple sequential treatments, more closely reflecting clinical approaches.
Shatsky R, Trivedi MS, Yau C, et al. Datopotamab–deruxtecan plus durvalumab in early-stage breast cancer: the sequential multiple assignment randomized I-SPY2.2 phase 2 trial. Nature Medicine https://www.nature.com/articles/s41591-024-03267-1
Khoury K, Meisel JL, Yau C, et al. Datopotamab deruxtecan in early stage breast cancer: the I-SPY2.2 sequential multiple assignment randomized phase 2 trial. Nature Medicine https://www.nature.com/articles/s41591-024-03266-2

Deborah’s breast cancer diagnosis in late 2022 catalyzed her interest in breast cancer research and patient advocacy following a complicated diagnosis. She is pleased to be participating as a patient advocate at UCSF, as well as with state, national and international groups, and has channeled her research and health care background in her advocacy work.
Deborah is a member of the National Breast Cancer Coalition and attended their Project Lead Science Training program in the summer of 2024, and will complete their Public Policy Institute in 2025. She serves on the Research Committee of the Lobular Breast Cancer Alliance (LBCA) and has been a grant reviewer for ASCO and LBCA. She begun working with Dr. Rita Mukhtar at UCSF on lobular breast cancer research and served on a panel for the Annual UCSF Breast Oncology Program Retreat. Deborah joined as a new member of the UCSF I-SPY Patient Advocate Panel in 2025 and serves as a Board member of Breast Cancer Care & Research Fund which links education, research and care to end breast cancer.
Throughout Deborah’s 30 plus year career in health care administration, she has worked to make health care more inclusive, accessible, equitable and personal. She started her health care career at the Stanford School of Medicine and then worked with several hospitals and health systems, including Kaiser Permanente where she worked for 22 years, serving as a medical group administrator and hospital CEO in Northern California, then at the National level as Vice President for Patient and Service Quality and as an Executive for National Quality and Board Governance.
Deborah is passionate about the importance of evidence-based research and multi-specialty team collaboration in solving problems and is committed to advancing breast cancer research, to supporting researchers, clinicians and patients, and to ensuring that the patients’ perspective is integrated into research, clinical trials and care. She has a BA with Highest Honors in Sociology and Research Methods from the University of California, Santa Cruz and a MBA from the Anderson School of Management at UCLA. She enjoys opera, jazz and the performing arts, as well as hiking, dancing and traveling, and is excited to be moving back to San Francisco in spring, 2025.

As Patient Engagement Lead at Quantum Leap, I manage follow-up data projects for the I-SPY 2 Trial, develop strategies to strengthen patient engagement, and oversee the I-SPY Advocate group. After six successful years raising funds to support Quantum’s mission, I embraced this new role last summer, drawn by the opportunity to make a direct impact on the patients we serve. Each day brings new challenges and insights, and I especially enjoy supporting and collaborating with our inspiring I-SPY Advocates.
Outside of work, I am pursuing a Master of Public Administration with a focus on nonprofit management at SF State. I’m passionate about trail running, skiing, live music, camping, traveling, and collegiate gymnastics—my daughter competes at Oregon State. For the past 5 years, I have volunteered with SF CASA as a mentor to an incredible 18-year-old foster youth whose resilience continually reminds me of life’s true priorities.

Carolyn Clark Beedle, a 2023 breast cancer survivor, joined the advocate program after successful completion of treatment with the UCSF Breast Oncology Program. Her experience working with a patient advocate during her TNBC treatment led to an understanding that empowering women to advocate for their own health and healthcare will contribute to improved health outcomes and broader access to care. Carolyn began advocating for cancer patients and their families during her treatment, now is a member of the Breast Science Advocacy Core (BSAC) with the UCSF Breast Oncology Program, and currently shares information and research with CFNP associates at La Clinica in the Bay Area to inform and empower their patient population.
Carolyn is continuing her on the ground training as an advocate reviewer with both Quantum Health and BSAC and is enrolled in the Patient Advocacy Training in Health Science course with Stanford Medicine. Her 30+ career in corporate marketing/communications, program development and non-profit leadership augmented by her breast cancer treatment experience serves Carolyn well in representing and communicating the patient voice and perspective.
She received her BA (History/English Lit) and MA (Public History/Research and Record Management) from the University of San Diego, is a proud fifth generation San Franciscan, and active board member with numerous non-profits that support social work and the arts.

Silver Alkhafaji is a PhD candidate in the Pharmaceutical Sciences and Pharmacogenomics (PSPG) program at UCSF. She received her Bachelor of Science in Chemical Biology from UC Berkeley. Prior to UCSF, she worked in the Clinical Pharmacology Department at Genentech. Silver’s current research focuses on non-invasive liquid biopsies to predict response and side effects of immunotherapies and endocrine therapies in early-stage breast cancer participants in I-SPY 2.
Silver is interested in clinical outcomes research to advance precision medicine and improve cancer patients’ quality of life. She is passionate about health equity, inclusive research, patient advocacy, and women’s health.
Silver volunteers at the Patient and Family Cancer Support Center at UCSF where she assists in patient navigation and connecting patients and their families with resources that improve their healthcare experience while receiving cancer treatments and/or during survivorship.
Through her DEI work in her PhD program, Silver raises awareness around issues related to social justice and community building through organizing community-centered events. Additionally, she is a member of the Life Sciences Career Advisory Council at Thrive Scholars, where she enjoys supporting college students of color from economically disadvantaged communities in providing the opportunities they need to thrive at top colleges and in high-trajectory careers.
Silver is a member of the American Association of University Women (AAUW) Alameda Branch where she focuses her efforts on increasing membership of community college women coming from exceptional backgrounds: student parents, low-income, and first-generation college students.
In her free time, she writes poetry and prose on emotional healing, radical acceptance, and patience. Writing has helped her process difficult situations and connect with people on a deeper level.

Jane is a breast cancer survivor and advocate dedicated to positively impacting the lives of women affected by the disease. Diagnosed with triple negative breast cancer in 2012, she participated in the I-SPY 2 trial at UCSF and has been cancer free for more than ten years.
Her advocacy journey began in 2003 at UCSF as a volunteer with the Patient and Family Cancer Support Center and Decision Support Services and she previously managed the Peer Support program at UCSF. Drawing on her experience in marketing and media strategy, she uses her skills to make a meaningful impact by supporting advocacy and research that improves outcomes for women living with breast cancer.

In March of 2023 , Jan was diagnosed with a large aggressive triple negative breast cancer and informed that her cancer was the” bad girl” of cancer and offered standard chemotherapy for 24 weeks. Devasted by the diagnosis Jan felt like she had a dire prognosis. After seeking several opinions, she opted to join a Clinical Trial program for her treatment. The trial consisted of significantly less chemotherapy, and monitored closely over a 12-week period, The data predicted a complete pathological response , and she then went immediately to surgery. Pathology reports supported that she had a successful outcome reaching PCR meaning the tumor was gone, and no residual cancer was found in the surrounding tissue or lymph nodes. Jan was thrilled when her surgeon advised her of the results. The experience made Jan want to give back and share information that she received when she was at a critical juncture in her diagnosis. She is so passionate about making sure that everyone knows that the standard of care is one treatment option.
As she says, “ clinical trials have to be on the table” Because she achieved PCR, she expects a great outcome. She wants to share her story and encourage other women to strongly consider and participate in clinical trials. Jan is a UCSF Patient Advocate, involved in several programs they lead. Jan also is a BLACC Cab Member. Jan recently was in Washington DC to participate on a panel on Clinical Trials for ISPY at the National Press Club. UCSF will be hosting the RISE Up For Breast Cancer event where Jan will share her experience with clinical trials.

Deb is a connector who founded Patient Advocates in Research (PAIR) “where research meets reality,” bringing ideas and people together for medical advances that offer real results for diverse patients and families.
Her vast experience between the worlds of tech, communication, strategy, management, policy, and equity bridges gaps between patients, scientists, medical providers, payers, governments, and non-profits.
Deb infuses patient engagement into projects, gathers relevant patient input, and encompasses many diseases, programs and policies at grassroots, national and international levels through companies, academia, and governments.
Key patient insights are delivered throughout discovery, development, clinical trials, results reporting, data-sharing, standards, genomics, and into practice.
Her experience spans translational and clinical research, epidemiology, health outcomes, and health delivery research with academia, federal agencies, companies, and patient communities.