Home › For Investigators › Meeting Abstracts
Meeting Abstracts
2022
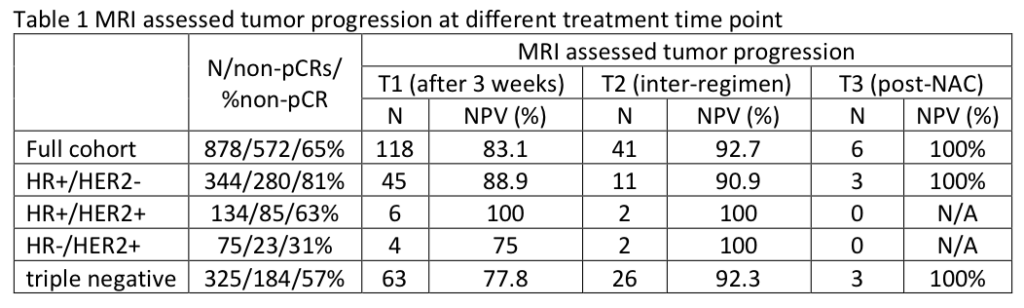
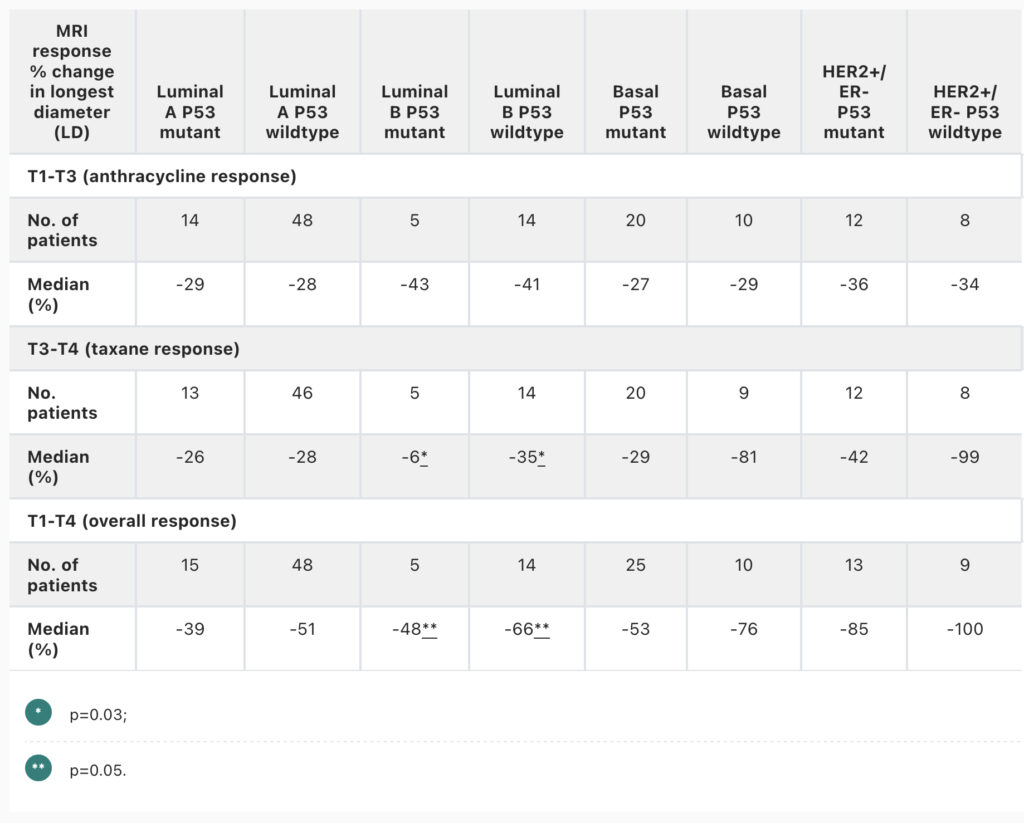
Zimmerman J, Carmona-Bozo J, Le NN, et al. Diffusion-weighted magnetic resonance imaging for subtype-specific prediction of pathologic complete response in neoadjuvant chemotherapy. 2022 San Antonio Breast Cancer Symposium, 8-11 Dec, 2022; Abstract No. P6-01-33.
Li W, Le NN, Onishi N, et al. Association of MRI morphologic phenotype from unsupervised learning with breast cancer subtypes and treatment response. 2022 San Antonio Breast Cancer Symposium, 8-11 Dec, 2022; Abstract No. PD16-07.
Onishi N, Jones EF, Carmona-Bozo J, et al. Early MRI and PET biomarkers for hormone receptor-positive/HER2-negative early-stage breast cancer in the setting of neoadjuvant endocrine therapy and neoadjuvant chemotherapy in the I-SPY 2 TRIAL. 2022 San Antonio Breast Cancer Symposium, 8-11 Dec, 2022; Abstract No. PD16-06.
Basu A, Umashankar S, Blevins K, et al. The Association Between Symptom Severity and Physical Function among Participants in I-SPY2. 2022 San Antonio Breast Cancer Symposium, 8-11 Dec, 2022; Abstract No. P5-07-03.
Magbanua MM, Rugo H, Brown-Swigart LA, et al. Monitoring for response and recurrence in neoadjuvant-treated hormone receptor-positive HER2-negative breast cancer by personalized circulating tumor DNA testing. 2022 San Antonio Breast Cancer Symposium, 8-11 Dec, 2022; Abstract No. P5-05-05
Li W, Onishi N, Wolf DM, et al. MRI models by response predictive subtype for predicting pathologic complete response. 2022 San Antonio Breast Cancer Symposium, 8-11 Dec, 2022; Abstract No. P4-02-10.
Rosenbluth J, Bui TB, Warhadpande S, et al. Characterization of residual disease after neoadjuvant selective estrogen receptor degrader (SERD) therapy using tumor organoids in the I-SPY Endocrine Optimization Protocol (EOP). 2022 San Antonio Breast Cancer Symposium, 8-11 Dec, 2022; Abstract No. P3-09-01.
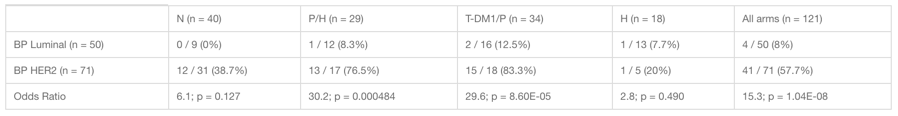
Wolf DM, Yau C, Wulfkuhle J, et al. Characterizing the HER2-/Immune-/DNA repair (DRD-) response predictive breast cancer subtype: the hunt for new protein targets in a high-needs population with low response to all I-SPY2 agents. 2022 San Antonio Breast Cancer Symposium, 8-11 Dec, 2022; Abstract No. PD5-04.
Bui TBV, Wolf DM, Moore K,et al. An Organoid Model System to Study Resistance Mechanisms, Predictive Biomarkers, and New Strategies to Overcome Therapeutic Resistance in Early-Stage Triple-Negative Breast Cancer. 2022 San Antonio Breast Cancer Symposium, 8-11 Dec, 2022; Abstract No. PD5-02.
Yee D, Shatsky RA, Yau C, et al. Improved pathologic complete response rates for triple-negative breast cancer in the I-SPY2 Trial. 2022 ASCO Annual Meeting, 3-7 Jun, 2022; Abstract No. 591.
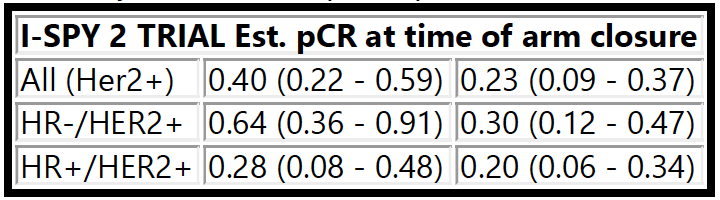
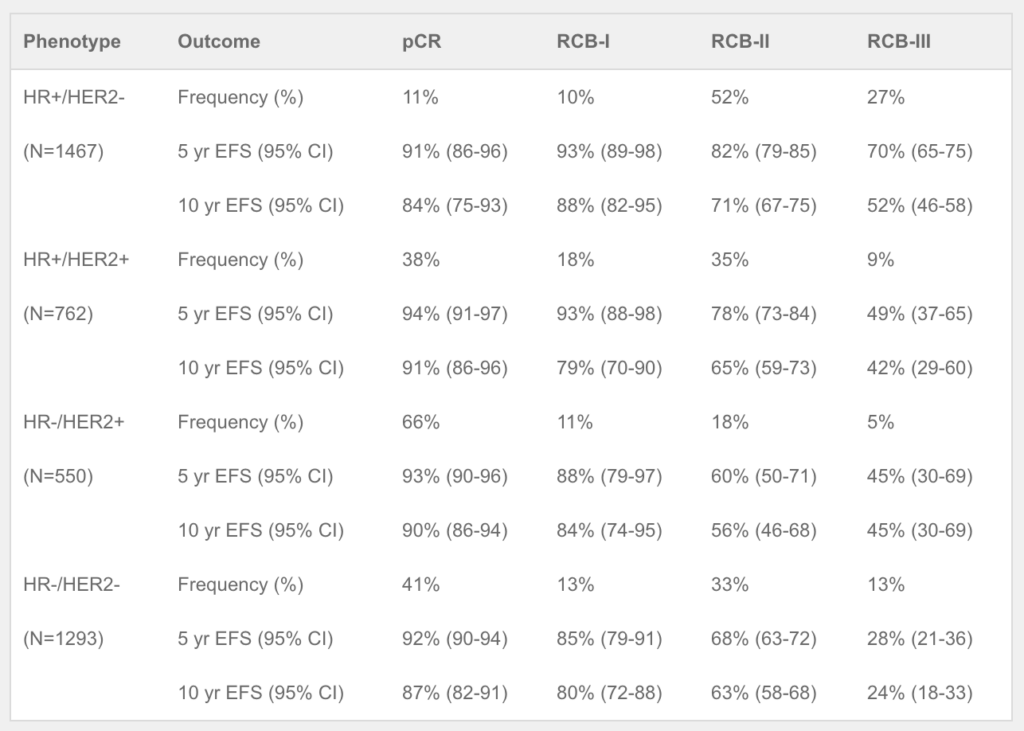
Thomas A, Clark AS, Yau C, et al. Molecular subtype to predict pathologic complete response in HER2-positive breast cancer in the I-SPY2 trial. 2022 ASCO Annual Meeting, 3-7 Jun, 2022; Abstract No. 510.
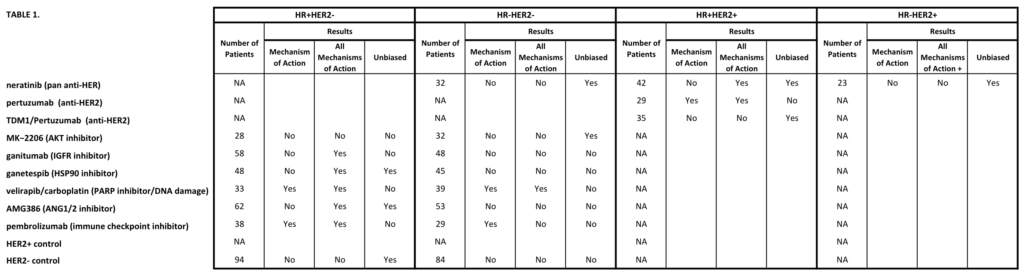
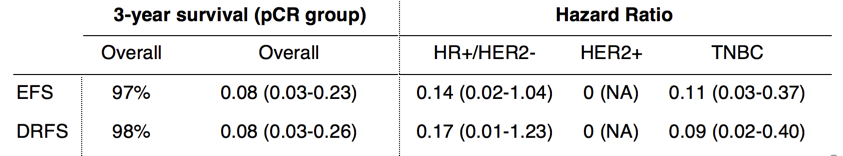
Huppert LA, Rugo HS, Pusztai L, et al. Pathologic complete response (pCR) rates for HR+/HER2- breast cancer by molecular subtype in the I-SPY2 Trial. 2022 ASCO Annual Meeting, 3-7 Jun, 2022; Abstract No. 504.
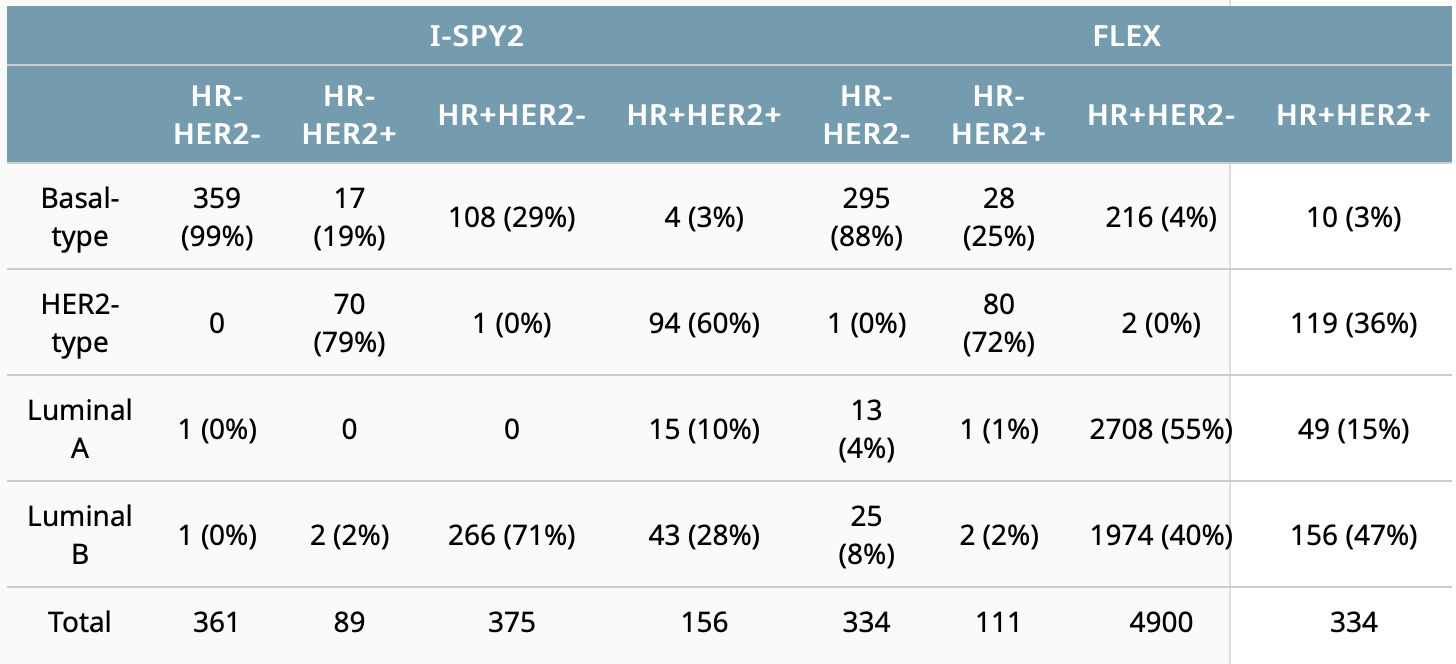
Cha J, Warner P, Hiatt R, et al. Distribution of breast cancer molecular subtypes within receptor classifications: Lessons from the I-SPY2 Trial and FLEX Registry. 2022 ASCO Annual Meeting, 3-7 Jun, 2022; Abstract No. 592.
Mittempergher L, Kuilman MM, Barcaru A, et al. The ImPrint immune signature to identify patients with high-risk early breast cancer who may benefit from PD1 checkpoint inhibition in I-SPY2. 2022 ASCO Annual Meeting, 3-7 Jun, 2022; Abstract No. 514
2021
Kyalwazi B, Yau C, Olopade O, et al. Analysis of clinical outcomes and expression-based immune signatures by race in the I-SPY 2 trial. 2021 San Antonio Breast Cancer Symposium, 7-10 Dec, 2021; Abstract No. GS4-02.
Northrop A, Christofferson A, Melisko M, et al. Improving patient-reported outcome data capture for clinical research: ePRO in ISPY 2, a phase 2 breast cancer study. 2021 San Antonio Breast Cancer Symposium, 7-10 Dec, 2021; Abstract No. P4-12-02.
Goodarzi H, Navickas A, Wang J, et al. Tumor-released circulating orphan non-coding RNAs reflect treatment response and survival in breast cancer. 2021 San Antonio Breast Cancer Symposium, 7-10 Dec, 2021; Abstract No. PD9-04.
Christofferson A, Price E, Mukhtar RA, et al. Single-institution retrospective analysis of lymph node (LN) change on breast MRI in patients with high risk early-stage breast cancer receiving neoadjuvant chemotherapy with and without immunotherapy on the ISPY-2 TRIAL. 2021 San Antonio Breast Cancer Symposium, 7-10 Dec, 2021; Abstract No. P3-03-07.
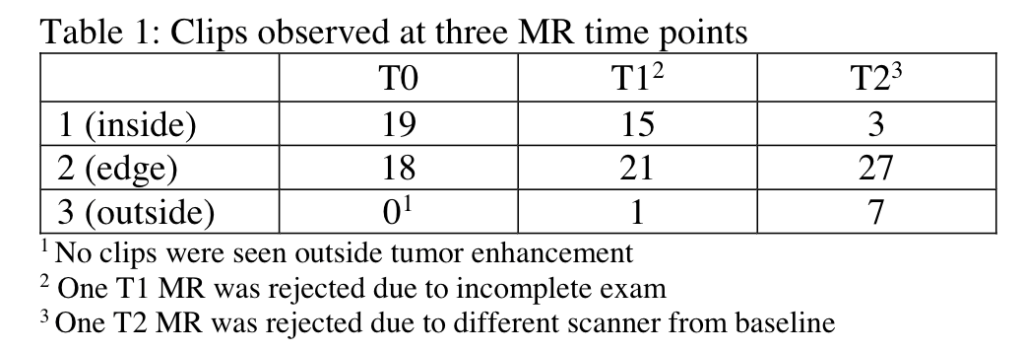
Bareng TJ, Gibbs JE, Onishi N, et al. Challenges of achieving high image quality on breast MRI for quantitative measurements in the I-SPY 2 TRIAL. 2021 San Antonio Breast Cancer Symposium, 7-10 Dec, 2021; Abstract No. P3-03-04.
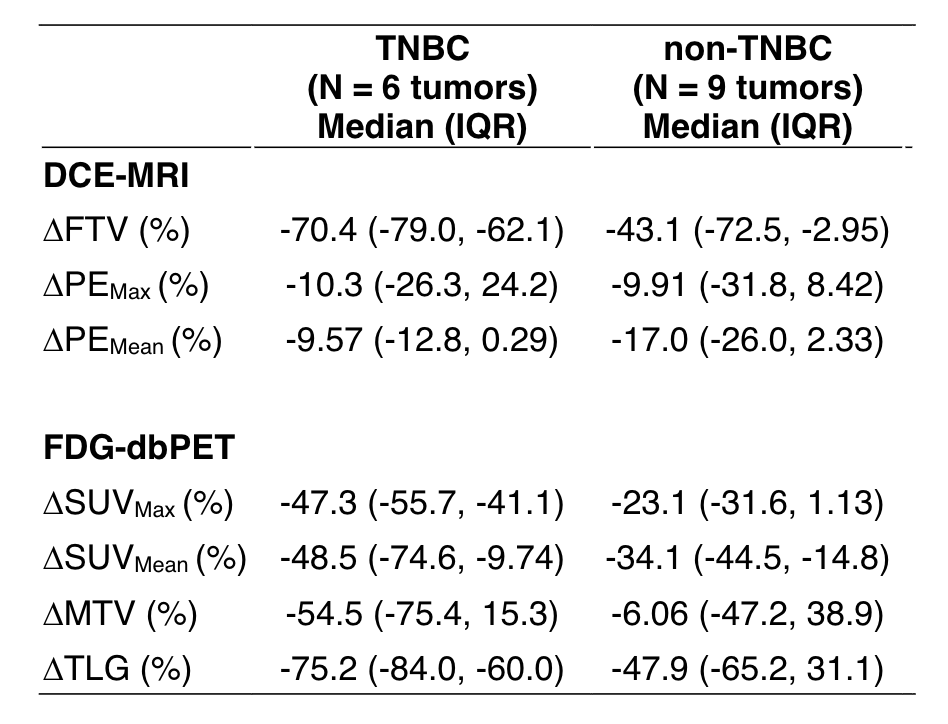
Li W, Le NN, Onishi N, et al. Diffusion-weighted MRI for prediction of pathologic complete response in HER2- breast cancer treated with pembrolizumab plus neoadjuvant chemotherapy. 2021 San Antonio Breast Cancer Symposium, 7-10 Dec, 2021; Abstract No. P3-03-02.
2020
Yee D, Haluska P, Wolf DM, et al. Biomarker analysis of paclitaxel, ganitumab, and metformin (PGM) therapy in the I-SPY2 neoadjuvant clinical trial. 2020 San Antonio Breast Cancer Symposium, Dec 8-11, 2020; Abstract No. PS4-08.
Wolf DM, Yau C, Brown-Swigart L, et al. Biomarkers predicting response to durvalumab combined with olaparib in the neoadjuvant I-SPY 2 TRIAL for high-risk breast cancer. 2020 San Antonio Breast Cancer Symposium, Dec 8-11, 2020; Abstract No. PD14-02.
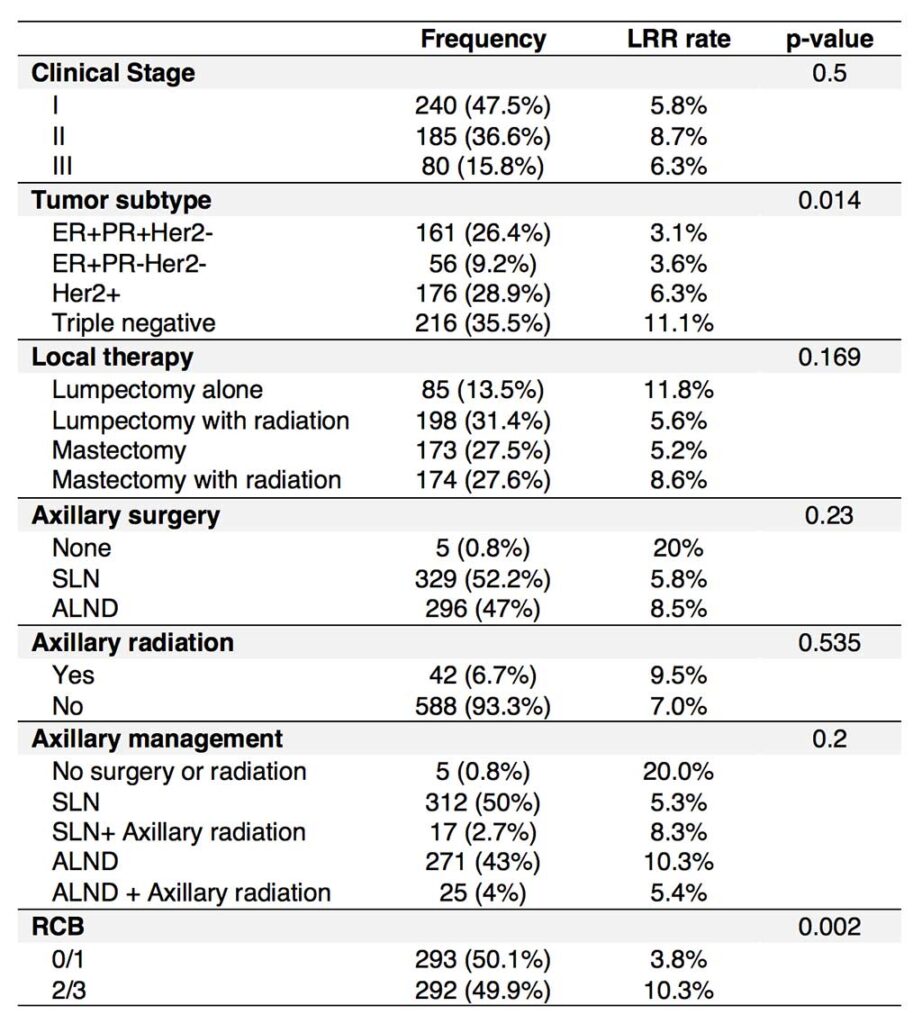
Shad S, van der Noordaa M, Osdoit M, et al. Site of recurrence after neoadjuvant therapy: a multi-center pooled analysis. 2020 San Antonio Breast Cancer Symposium, Dec 8-11, 2020; Abstract No. PD13-02.
van der Noordaa MEM, Yau C, Shad S, et al. Assessing prognosis after neoadjuvant therapy: A comparison between anatomic ypAJCC staging, Residual Cancer Burden Class and Neo-Bioscore. 2020 San Antonio Breast Cancer Symposium, Dec 8-11, 2020; Abstract No. GS4-07.
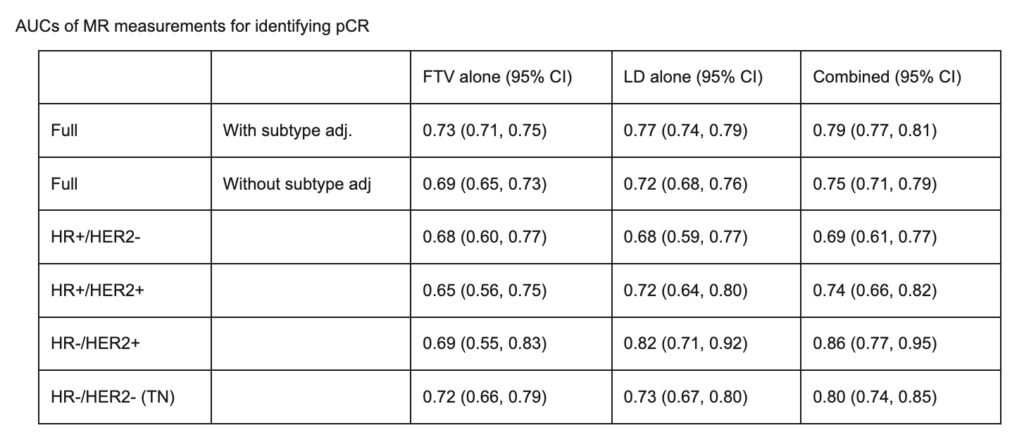
Li W, Newitt DC, Gibbs J, et al. Subtype-specific MRI models to guide selection of candidates for de-escalation of neoadjuvant therapy. 2020 San Antonio Breast Cancer Symposium, Dec 8-11, 2020; Abstract No. PD6-05.
Magbanua MJM, Wolf D, Renner D, et al. Personalized circulating tumor DNA (ctDNA) as predictive biomarker in high-risk early stage breast cancer (EBC) treated with neoadjuvant chemotherapy (NAC) with or without pembrolizumab (P). 2020 San Antonio Breast Cancer Symposium, Dec 8-11, 2020; Abstract No. PD9-02.
2019
Pohlmann PR, Yau C, DeMichele A, et al. Amsterdam 70-gene profile (MammaPrint) Low Risk, even in the HER2 positive subset, identifies a population of women with lower early risk for recurrence despite low response rates to chemotherapy and to HER2 targeted therapy. 2019 San Antonio Breast Cancer Symposium, December 10-14, 2019; Abstract No. P6-10-06.
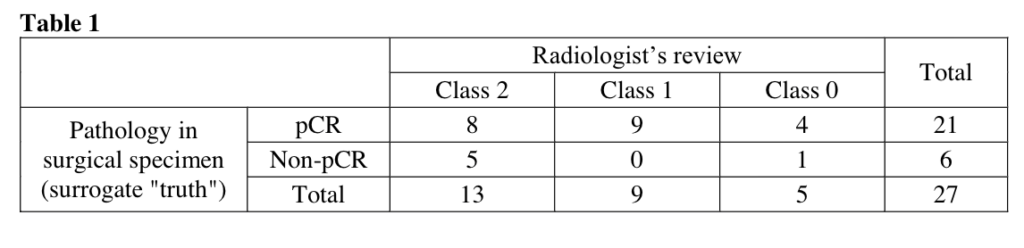
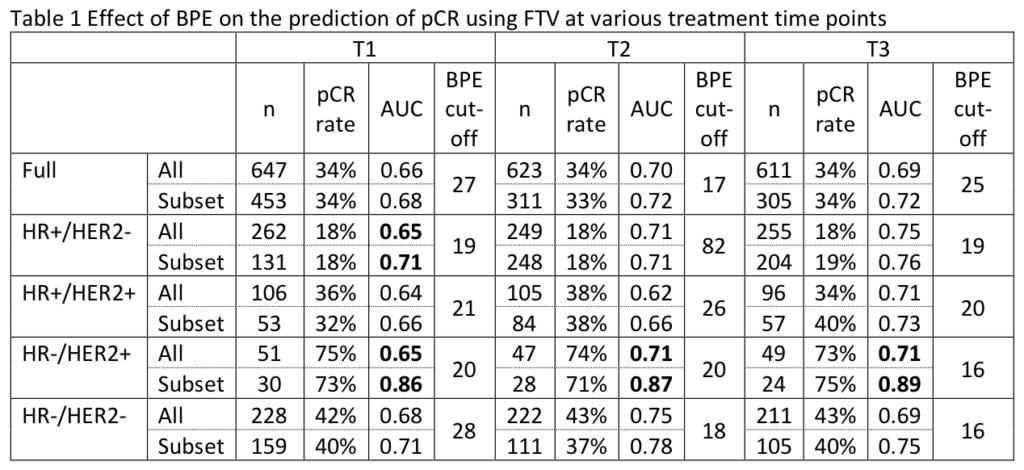
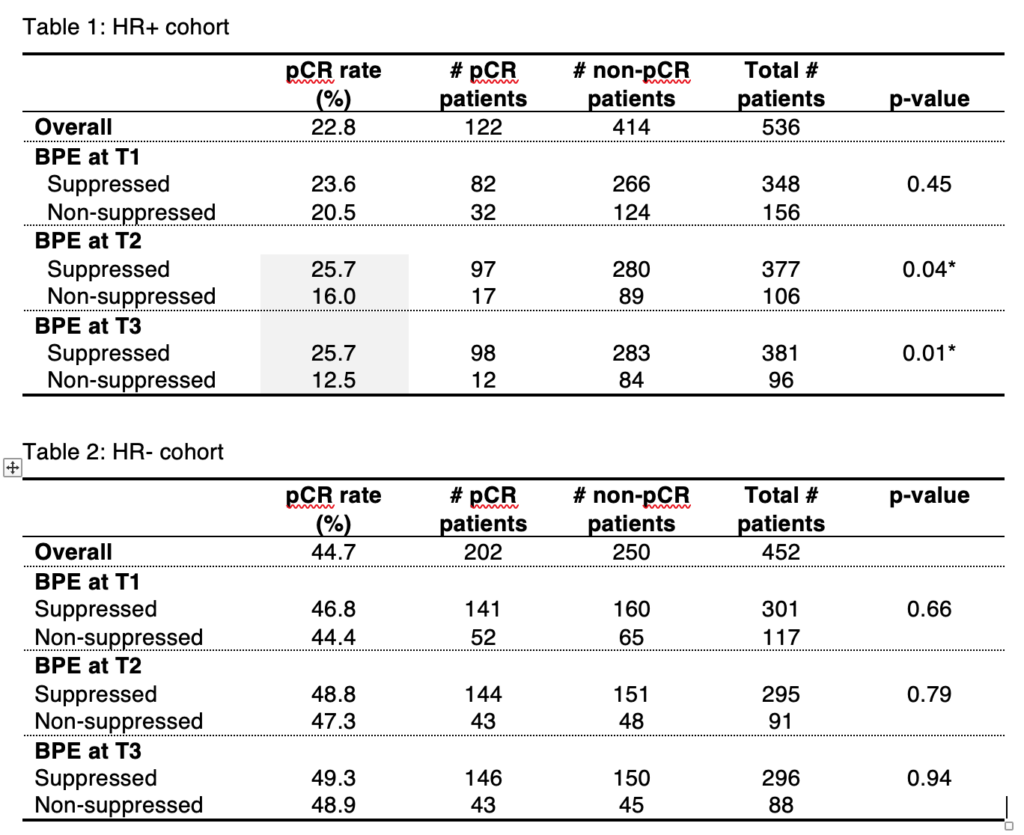
Li W, Onishi N, Newitt DC, et al. The effect of background parenchymal enhancement on the predictive performance of functional tumor volume measured in MRI. 2019 San Antonio Breast Cancer Symposium, December 10-14, 2019; Abstract No. P6-02-01.
Venters SJ, Wolf DM, Brown-Swigart L, et al. Assessing biomarkers to inform treatment de-escalation: mid-treatment biopsy cellularity predicts pCR in the I-SPY 2 TRIAL. 2019 San Antonio Breast Cancer Symposium, December 10-14, 2019; Abstract No. P6-10-02.
Magbanua MM, Brown-Swigart L, Hirst G, et al. Personalized monitoring of circulating tumor DNA during neoadjuvant therapy in highrisk early stage breast cancer reflects response and risk of metastatic recurrence. 2019 San Antonio Breast Cancer Symposium, December 10-14, 2019; Abstract No. P5-01-04.
Ghersin H, Chattopadhyay A, Blaes A, et al. Introducing an electronic platform to collect patient reported outcomes in the I-SPY 2 TRIAL, a neoadjuvant clinical trial. 2019 San Antonio Breast Cancer Symposium, December 10-14, 2019; Abstract No. OT3-19-02.
Yau C, van der Noordaa M, Wei J, et al. Residual cancer burden after neoadjuvant therapy and long-term survival outcomes in breast cancer: a multi-center pooled analysis. 2019 San Antonio Breast Cancer Symposium, December 10-14, 2019; Abstract No. GS5-01.
2018
Hyland C, Varghese F, Yau C, et al. The use of 18F-FDG PET/CT as an initial staging procedure for stage II-III breast cancer reduces false positives, costs, and time to treatment: a multicenter value analysis in the I-SPY2 trial. 2018 San Antonio Breast Cancer Symposium, December 4-8, 2018; Abstract No. P5-15-01.