Home › For Investigators › RECAST DCIS
RECAST DCIS
The Re-Evaluating Conditions for Active Surveillance Suitability as Treatment: Ductal Carcinoma In Situ (RECAST DCIS) Platform Trial is a landmark Phase 2 trial that is changing the lens through which we treat DCIS. The study is aimed at preventing the progression of DCIS to breast cancer and is evaluating three investigational endocrine therapy arms.
DCIS Treatment, Re-Imagined



Atossa’s (Z)-endoxifen
Havah Therapeutics' HAVAH T+Ai™
Stemline’s Elacestrant (ORSERDU)
Stemline’s Elacestrant (ORSERDU) is a selective estrogen receptor degrader, taken orally once daily as a tablet. It is an antagonist that selectively binds to ERa and acts to inhibit estrogen receptor expression via degradation and estradiol-mediated cell proliferation. ORSERDU was approved by the U.S. Food & Drug Administration in January 2023 for the treatment of post-menopausal women or adult men with estrogen receptor (ER)-positive, HER2-negative, ESR-1 mutated advanced or metastatic breast cancer, following at least one line of endocrine therapy. For more information about prescribing guidelines, please visit https://www.orserdu.com/

RECAST DCIS Study Schema
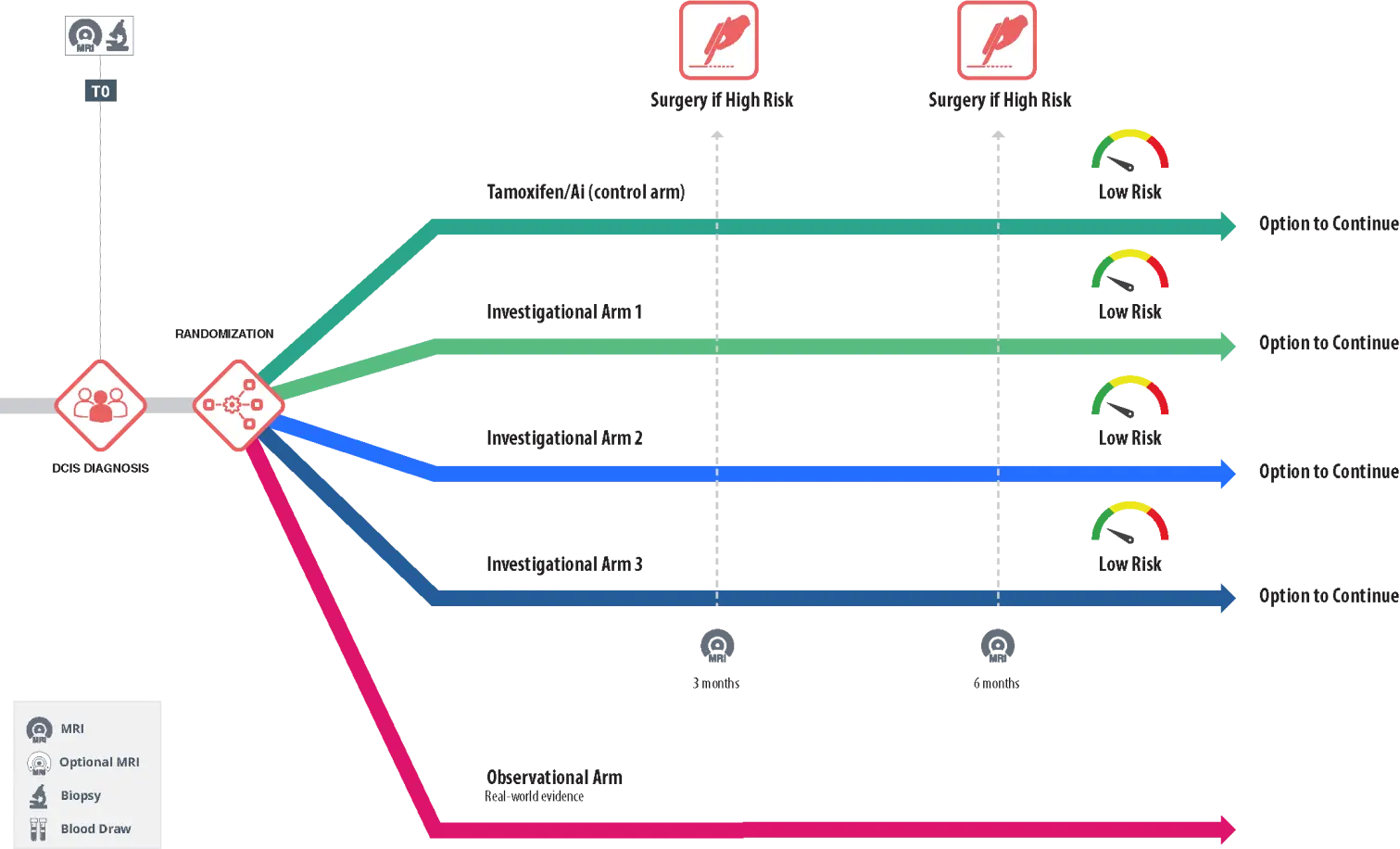
Download a List of
RECAST DCIS Trial Sites
RECAST DCIS Trial Sites
Adobe PDF (515kb)












