Home › For Patients › I-SPY Trials › I-SPY Trial Locations
Trial Locations
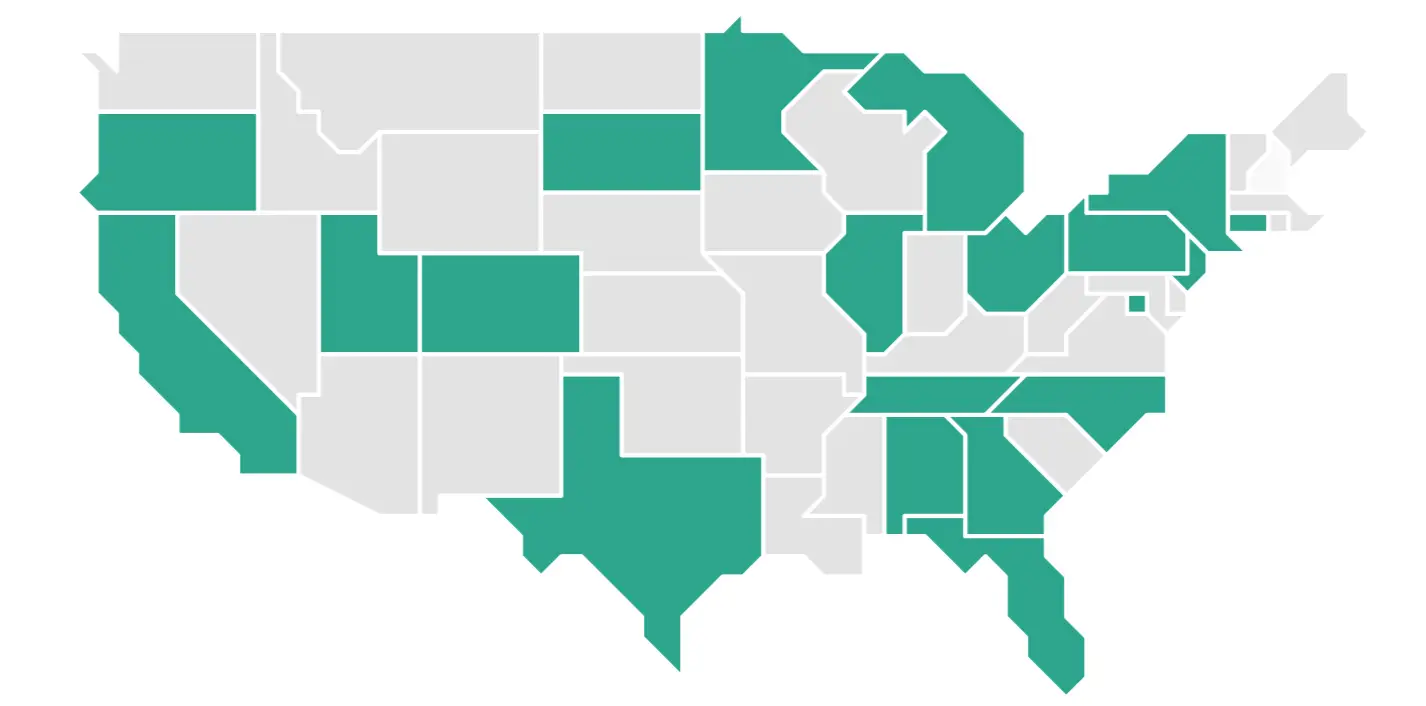
The I-SPY 2 Trial is taking place at about 50 sites across the United States. Check back frequently because new study sites open all the time.
Use this interactive tool to find the I-SPY 2 Trial location nearest to you. This takes you to contact information for the study site so you can get answers to any additional questions you have or express your interest in participating in the study. If there is no Clinical Trial Site near you, consider traveling to participate in the I-SPY 2 Trial.
Click on a state below to view study site contact information
Oregon
Oregon Health and Science University Knight Cancer Institute
Portland, OR 97239
Alexandra Zimmer, MD
trials@ohsu.edu
503-494-1080, 503-494-7999 (appointment)
https://apps.ohsu.edu/research/study-participation-opportunities-system/knight_viewstudy.php?id=IRB00006256 (I-SPY)
California
City of Hope
Irvine, CA 92618
Jennifer Tseng, MD
https://www.cityofhope.org/patients
800-826-4673
Hoag Family Cancer Institute
Newport Beach, CA 92663
Chaitali Nangia, MD
https://www.hoag.org/contact-us/appointments/
University of California, San Francisco Carol Franc Buck Breast Care Center
San Francisco, CA 94143
Amy Jo Chien, MD
415-353-9579 – New Patient Coordinator
https://clinicaltrials.ucsf.edu/trial/NCT01042379
University of California San Diego Moores Cancer Center
La Jolla, CA 92093
Anne Wallace, MD
866-773-2703 (clinic appts must go through clinician to be referred to I-SPY)
https://clinicaltrials.ucsd.edu/trial/NCT01042379
University of Southern California Norris Comprehensive Cancer Center
Los Angeles CA 90033
Evanthia Roussos Torres, MD, PhD
clinical.trials@med.usc.edu
323-865-0451 (clinical investigation support office)
323-865-3111 (new patient referrals)
https://uscnorris.com/CLTrials/ViewProtocol.aspx?protocol_num=1B-10-3&protocol_id=2497
University of California, Davis Comprehensive Cancer Center
Davis, CA 95817Mili Arora, MD
916-734-5959 or 800-770-9261 (new patient referral)
916-734-3604 (clinical trials info)
Teri Nguyen (coordinator) 916-734-8266
Utah
University of Utah, Huntsman Cancer Institute
Salt Lake City, Utah, 84112
Christos Vaklavas, MD
1-888-424-2100 (cancer learning center, will direct to CRCs)
https://healthcare.utah.edu/huntsmancancerinstitute/clinical-trials/trial.php?id=27388&no=137669
Colorado
University of Colorado Cancer Center
Aurora, CO 80045
Virginia Borges, MD
researchstudies@cuanschutz.edu
720-848-0408 (breast cancer clinic)
South Dakota
Sanford Clinical Research
Sioux Falls, SD, 57104
Amy Sanford, MD
605-328-8000
605-328-1368
Texas
Coming Soon
Mays Cancer Center
San Antonio, TX
Minnesota
University of Minnesota Masonic Cancer Center
Minneapolis, MN, 55455
Douglas Yee, MD
ccinfo@umn.edu
612-624-2620 – Nurse Navigator Line for more info on clinical trials that may be available to you
612-676-4200 – Breast Cancer Clinic appointments
https://clinicaltrial.cancer.umn.edu/sip/#
Mayo Clinic Breast Cancer Center–Rochester
Rochester, MN 55905
Judy C. Boughey, MD
855-776-0015
https://www.mayo.edu/research/clinical-trials/cls-20116766#eligibility
https://www.mayo.edu/research/forms/cancer-clinical-trials?pocid=CLS-20116766&nctid=NCT01042379&epicenterid=E10014713
Michigan
Sparrow
Lansing, MI 48192
Brittani Thomas, MD
1.800.Sparrow (1.800.772.7769)
https://www.sparrow.org/departments-conditions/all-departments/cancer-care-oncology
Illinois
Loyola University Cardinal Bernardin Cancer Center
Maywood, IL 60153
Kathy S. Albain, MD
mbartolotta@luc.edu
888-584-7888
https://loyolamedicine.org/cancer/breast-cancer (to make an appointment)
University of Chicago Medical Center
Chicago, IL 60437
Rita Nanda, MD
cancerclinicaltrials@bsd.uchicago.edu
1-855-702-8222
https://www.uchicagomedicine.org/find-a-clinical-trial/clinical-trial/6/10183b
Ohio
Cleveland Clinic Taussig Cancer Center
Cleveland, OH 44106
Erin Roesch, MD
800-233-2273
https://my.clevelandclinic.org/clinical-trials/1485-i-spy-2-trial—investigation-of-serial-studies-to-predict-your-therapeutic-response-with-imaging-and-molecular-analysis-2
Ohio State University Comprehensive Cancer Center-The James
Columbus, OH 43210
Nicole Williams, MD
herohelpline@osu.edu (clinical trial help email)
614-293-4376 (HERO) – inquire about cancer clinical trials
614-293-5066 or toll-free at 800-293-5066 to make an appointment
Pennsylvania
University of Pennsylvania Abramson Cancer Center
Philadelphia, PA 19104
Amy Clark, MD, MSCE
1-855-216-0098
https://app.emergingmed.com/acc-upenn/trial/46485/
Washington DC
Georgetown University Lombardi Cancer Center
Washington, DC 20007
Claudine Isaacs, MD
202-444-2223 (make an appointment)
https://lombardi.georgetown.edu/patient/info/schedule/# (make an appointment)
New York
Colombia Herbert Irving Comprehensive Cancer Center
New York, New York
Meghna Trivedi, MD
cancerclinicaltrials@cumc.columbia.edu
212-305-5098 (new patient navigation line)
212-342-5162 (clinical trial navigator line)
https://recruit.cumc.columbia.edu/clinical_trial/1371
https://cumc.co1.qualtrics.com/jfe/form/SV_est8ZlfAcxfnnyB (New Patient Form)
University of Rochester Medicine Wilmot Cancer Institute
Rochester, NY 14642
Carla Falkson, MD
1-866-494-5668 (4WILMOT)
Email: WCICTOResearch@urmc.rochester.edu
New Jersey
Rutgers Cancer Institute of New Jersey
New Brunswick, NJ 08901
Coral Omene MD
732-235-7356
https://cinj.org/clinical-trials/find-clinical-trial?show=trial&p=042102
Rutgers Saint Barnabas Medical Center
Livingston NJ, 07039
(973) 322-5000
Rutgers Robert Wood Johnson University Hospital Somerset
Somerville, NJ 08876
732-235-7356 or 844-CANCERNJ
Connecticut
Yale Medical Center
New Haven, CT 05620
Mariya Rozenblit, MD
helpusdiscover@yale.edu
203-200-2328 (breast center)
877-978-8343 (clinical trials)
Tennessee
Vanderbilt-Ingram Cancer Center
Nashville, TN 27204
Ingrid Mayer, MD
1-800-811-8480 (learn about clinical trials)
615-936-8422
https://www.vicc.org/clinical-trials/protocol-viccbre1951
North Carolina
Wake Forest Baptist Health Comprehensive Cancer Center
Winston-Salem, NC 27157
Alexandra Thomas, MD, FACP
btopalog@wakehealth.edu
1-833-206-1202 (clinical trials)
336-716-0248 (ISPY)
336-716-WAKE (9253)
888-716-9253 (toll-free) (appointments)
https://www1.wakehealth.edu/beinvolved/eng/studies/IRB00053064
Alabama
University of Alabama at Birmingham Comprehensive Cancer Center
Birmingham, AL 35294
Erica Stringer-Reasor
205-801-9034 (make an appointment with cancer center)
https://www.uabmedicine.org/appointment-cancer (appointment)
Georgia
Emory University Winship Cancer Institute
Atlanta, GA 30322
Kevin Kalinsky, MD, MS
winshipcto@emory.edu
https://winshipcancer.emory.edu/patient-care/being-a-patient/index.html (to request an appointment)
404-778-1868
404-778-PINK (778-7465) to request an appointment
Coming Soon
Emory St Joseph’s
Atlanta, GA 30342
Florida
Moffitt Cancer Center
Tampa, FL 33612
Heather Han, MD
813-745-6100 or 1-800-679-0775 (clinical trial numbers)
1-888-663-3488 (make an appointment)
https://moffitt.org/patient-family/preparing-for-your-appointment/scheduling-your-appointment/ (make an appointment)
List of All Trial Locations
Alabama
University of Alabama at Birmingham Comprehensive Cancer Center
Birmingham, AL 35294
Erica Stringer-Reasor
205-801-9034 (make an appointment)
uabmedicine.org (appointments)
California
City of Hope
Irvine, CA 92618
Jennifer Tseng, MD
https://www.cityofhope.org/patients
800-826-4673
Hoag Family Cancer Institute
Newport Beach, CA 92663
Chaitali Nangia, MD
www.hoag.org
University of California, San Francisco Carol Franc Buck Breast Care Center
San Francisco, CA 94143
Amy Jo Chien, MD
415-353-9579 – New Patient Coordinator
clinicaltrials.ucsf.edu
University of California San Diego Moores Cancer Center
La Jolla, CA 92093
Anne Wallace, MD
866-773-2703 (clinic appts must go through clinician to be referred to I-SPY)
clinicaltrials.ucsd.edu
University of Southern California Norris Comprehensive Cancer Center
Los Angeles CA 90033
Evanthia Roussos Torres, MD, PhD
clinical.trials@med.usc.edu
323-865-0451 (clinical investigation support office)
323-865-3111 (new patient referrals)
uscnorris.com
University of California, Davis Comprehensive Cancer Center
Davis, CA 95817Mili Arora, MD
916-734-5959
or 800-770-9261 (new patient referral)
916-734-3604 (clinical trials info)
Teri Nguyen (coordinator)
916-734-8266
Colorado
University of Colorado Cancer Center
Aurora, CO 80045
Virginia Borges, MD
researchstudies@cuanschutz.edu
720-848-0408 (breast cancer clinic)
Connecticut
Yale Medical Center
New Haven, CT 05620
Mariya Rozenblit, MD
helpusdiscover@yale.edu
203-200-2328 (breast center)
877-978-8343 (clinical trials)
Florida
Moffitt Cancer Center
Tampa, FL 33612
Heather Han, MD
813-745-6100
1-800-679-0775 (clinical trial numbers)
1-888-663-3488 (make an appointment)
moffitt.org (make an appointment)
Georgia
Emory University Winship Cancer Institute
Atlanta, GA 30322
Kevin Kalinsky, MD, MS
winshipcto@emory.edu
winshipcancer.emory.edu (to request an appointment)
404-778-1868
404-778-PINK (778-7465) to request an appointment
Illinois
Loyola University Cardinal Bernardin Cancer Center
Maywood, IL 60153
Kathy S. Albain, MD
mbartolotta@luc.edu
888-584-7888
loyolamedicine.org ( appointments)
University of Chicago Medical Center
Chicago, IL 60437
Rita Nanda, MD
cancerclinicaltrials@bsd.uchicago.edu
1-855-702-8222
www.uchicagomedicine.org
Michigan
Sparrow
Lansing, MI 48192
Brittani Thomas, MD
1.800.Sparrow (1.800.772.7769)
www.sparrow.org
Minnesota
University of Minnesota Masonic Cancer Center
Minneapolis, MN, 55455
Douglas Yee, MD
ccinfo@umn.edu
612-624-2620 – Nurse Navigator Line for more info on clinical trials that may be available to you
612-676-4200 – Breast Cancer Clinic appointments
clinicaltrial.cancer.umn.edu
Mayo Clinic Breast Cancer Center–Rochester
Rochester, MN 55905
Judy C. Boughey, MD
855-776-0015
www.mayo.edu
www.mayo.edu/research
New Jersey
Rutgers Saint Barnabas Medical Center
Livingston NJ, 07039
(973) 322-5000
Rutgers Robert Wood Johnson University Hospital Somerset
Somerville, NJ 08876
732-235-7356
or 844-CANCERNJ
University of Pennsylvania Abramson Cancer Center
Philadelphia, PA 19104
Amy Clark, MD, MSCE
1-855-216-0098
app.emergingmed.com/…
New York
Colombia Herbert Irving Comprehensive Cancer Center
New York, New York
Meghna Trivedi, MD
cancerclinicaltrials@cumc.columbia.edu
212-305-5098 (new patient navigation line)
212-342-5162 (clinical trial navigator line)
recruit.cumc.columbia.edu
cumc.co1.qualtrics.com (New Patient Form)
University of Rochester Medicine Wilmot Cancer Institute
Rochester, NY 14642
Carla Falkson, MD
1-866-494-5668 (4WILMOT)
Email: WCICTOResearch@urmc.rochester.edu
North Carolina
Wake Forest Baptist Health Comprehensive Cancer Center
Cleveland, OH 44106
Erin Roesch, MD
800-233-2273
my.clevelandclinic.org
Ohio
Cleveland Clinic Taussig Cancer Center
Winston-Salem, NC 27157
Alexandra Thomas, MD, FACP
btopalog@wakehealth.edu
1-833-206-1202 (clinical trials)
336-716-0248 (ISPY)
336-716-WAKE (9253)
888-716-9253 (toll-free) (appointments)
www1.wakehealth.edu
Ohio State University Comprehensive Cancer Center-The James
Columbus, OH 43210
Nicole Williams, MD
herohelpline@osu.edu (clinical trial help email)
614-293-4376 (HERO) – inquire about cancer clinical trials
614-293-5066 or toll-free at 800-293-5066 to make an appointment
Pennsylvania
University of Pennsylvania Abramson Cancer Center
Philadelphia, PA 19104
Amy Clark, MD, MSCE
1-855-216-0098
app.emergingmed.com/…
South Dakota
Sanford Clinical Research
Sioux Falls, SD, 57104
Amy Sanford, MD
605-328-8000
605-328-1368
Utah
University of Utah, Huntsman Cancer Institute
Salt Lake City, Utah, 84112
Christos Vaklavas, MD
1-888-424-2100 (cancer learning center, will direct to CRCs)
healthcare.utah.edu
Washington DC
Georgetown University Lombardi Cancer Center
Washington, DC 20007
Claudine Isaacs, MD
202-444-2223 (make an appointment)
lombardi.georgetown.edu (make an appointment)
Salt Lake City, Utah, 84112
Christos Vaklavas, MD
1-888-424-2100 (cancer learning center, will direct to CRCs)
healthcare.utah.edu
Consider traveling to an I-SPY Trial
If there is no Clinical Trial Site near you, consider traveling to participate in the I-SPY 2 Trial. Listen to Barri discuss why she decided to travel to an I-SPY 2 Clinical Trial site to participate.













