Home › For Patients › I-SPY Trials › Post June 23, 2022
Welcome to the I-SPY 2 Trial website for patients
The I-SPY 2 Trial is an investigational drug trial for newly diagnosed patients with locally advanced breast cancer. An investigational drug has been approved for testing in people by the U.S. Food and Drug Administration (FDA) but has not yet been approved for widespread use. A clinical trial tests how well investigational drugs work and whether they are safe to use.
- Please watch this 2-minute I-SPY 2 Trial introduction video to get a quick overview of the trial.
- Then find all the information you need to decide whether you want to be screened to participate in the trial.
- The National Cancer Institute’s electronic dictionary of common cancer terms is at the bottom of every page so you can look up words that are unfamiliar to you.
The I-SPY 2 Trial has three main phases
Screening, Treatment and Follow Up
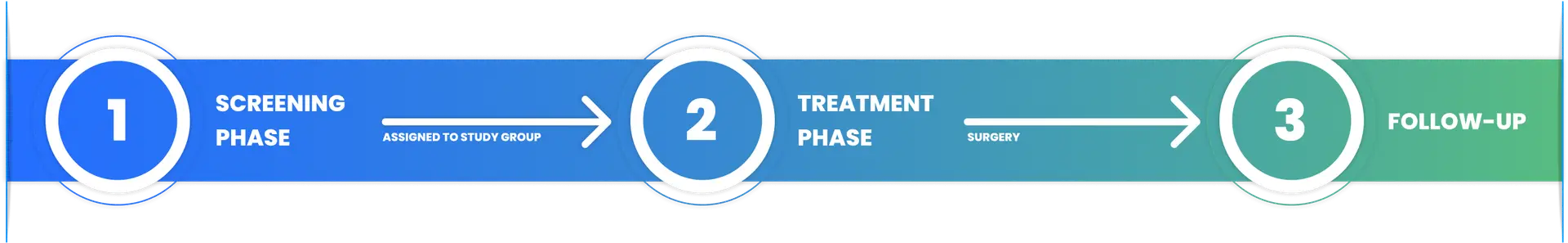
Who can be screened for I-SPY 2 Trial eligibility?
Women and men who are:
- At least 18 years old
- Have a new diagnosis of Stage 2 or 3 invasive breast cancer
- Have a tumor 2.5cm (almost an inch) or larger
If you meet these requirements, no matter what type of breast cancer you have and whether or not there is cancer in your lymph nodes, you are eligible to be screened for participation in the I-SPY 2 Trial.
During the Screening Phase your study doctor will check to make sure it is safe for you to take part in the study. This includes checking your heart, liver and kidney function. You will have a biopsy (tissue sample of your tumor) and a test called MammaPrint®. MammaPrint is a test to find out if your cancer would be at high risk for recurrence if the only treatment you had for your cancer was surgery.
Who can continue on to the Treatment Phase of the I-SPY 2 Trial?
- are otherwise healthy
- have not had cancer within the last 5 years*
- have not had chemotherapy for this breast cancer
- are not pregnant or breastfeeding
* You may still participate if you have had breast cancer, carcinoma in situ of the cervix, colon or rectum, melanoma in situ, basal cell and squamous cell carcinomas of the skin, or papillary thyroid cancer within the last 5 years.
Please watch this video to learn more about the trial.
Introduction to the I-SPY 2 Trial
Laura Esserman, MD, MBA
Surgeon Director, Carole Franc Buck Breast Care Center, University of California, San Francisco I-SPY 2 Clinical Trial, Prinicipal Investigator
- :00 Introduction to I-SPY 2 Trial
- 2:49 Screening for I-SPY 2
- 4:26 Breast cancer is not an emergency
- 5:44 Purpose of I-SPY
- 6:32 Understanding your tumor
- 7:16 Your treatment on I-SPY 2 Trial
- 10:10 Adjusting therapy based on response
- 12:54 Your symptoms and your voice matter
- 14:08 Thank you and conclusion
Why consider joining the I-SPY 2 Trial?
The I-SPY 2 Trial treats newly diagnosed breast cancer with neoadjuvant drug therapy. This means that drug therapy is given before surgery. Neoadjuvant therapy has advantages and disadvantages. One advantage is that it may shrink your tumor, allowing you to have a smaller surgery. One disadvantage is that you have to wait until you have finished neoadjuvant therapy to have your surgery, which may be stressful for some people. However, it is just as safe and effective to have therapy before surgery as after surgery. You can learn more about the pros and cons of neoadjuvant therapy here.
The I-SPY 2 Trial is testing new investigational drugs and combinations of drugs to find out whether they are better, worse or no different than the standard treatment. Most patients on the trial receive an investigational drug or drug combination that would not be available to them as standard treatment.
The I-SPY 2 Trial uses 3D MRIs to monitor your tumor. MRI (Magnetic Resonance Imaging) scans provide you and your care team with detailed pictures of your tumor. Your doctor can see if your tumor is responding to the drugs or not.
The I-SPY 2 Trial measures biomarkers (characteristics of your tumor) to learn as much as possible about your breast cancer and how it responds to treatment. A small part of your tumor will be collected by biopsy. Part of this biopsy will be used for molecular testing. These tests may give you and your doctor more information about your tumor, which will help with planning your treatment. As you learned in the videos above, I-SPY 2 is using a new biomarker called the Response Predictive Subtype (RPS). RPS helps predict if your tumor may respond to immune oncology drugs or other types of drugs, such as PARP inhibitors. (Use the NIH Dictionary of Cancer Terms at the bottom of the page to learn more about PARP inhibitors). This also enables you to have a greater chance of being treated with a drug that is targeted to your tumor’s biology.
Please watch these two videos to better understand how your breast cancer is evaluated and treated on the I-SPY 2 Trial.
Introduction to the I-SPY 2 Trial
Angela DeMichele, MD, MSCE
Alan and Jill Miller Professor in Breast Cancer Excellence Perelman Center for Advanced Medicine University of Pennsylvania I-SPY 2 Principal Investigator
- :00 Introduction to the video
- :52 What is the I-SPY 2 Trial
- 2:14 Informed Consent process
- 3:16 Screening for eligibility to participate
- 4:40 What is a Functional Tumor Volume MRI?
- 6:36 Response Predictive Subtypes and the I-SPY Biopsy
- 7:37 Your treatment on I-SPY 2 Trial
- 10:40 Shared Test Results and Shared Decision Making
- 11:20 Treatment Strategy of I-SPY 2
- 13:55 How the MRI is used in I-SPY 2
- 17:03 Deciding whether to go to surgery early
- 18:35 Summary of the video
Length: 18:35 min
- Estrogen Receptor (ER) status, Progesterone Receptor (PR) status, and HER2 receptor status are common biomarkers used to characterize a breast cancer.
- These biomarkers will be used, along with the MammaPrint test score to help determine whether you are eligible to join the Chemotherapy or the Endocrine Treatment Phase of the trial.
You are eligible to continue onto the Chemotherapy Treatment Phase, if your cancer matches one of these descriptions:
Estrogen Receptor Positive & MammaPrint® High Risk
You are eligible to continue onto the Endocrine Treatment Phase, if your cancer matches this description:
*As you learned in the videos above, RPS (Response Predictive Subtype) is a molecular test that predicts your tumor’s response to certain treatments.
SET (Sensitivity to Endocrine Therapy) is a molecular test that predicts your tumor’s sensitivity to endocrine therapy.
You may choose either the Chemotherapy or Endocrine Treatment Phase if your tumor is Estrogen Receptor positive, HER2 negative & MammaPrint high Risk and if your tumor is RPS negative.
You can find more information about the Endocrine Treatment Phase here.
What to expect in each phase of the trial
The timeline below shows the activities that occur during each phase of the trial,
depending on whether you are receiving Chemotherapy or Endocrine therapy.
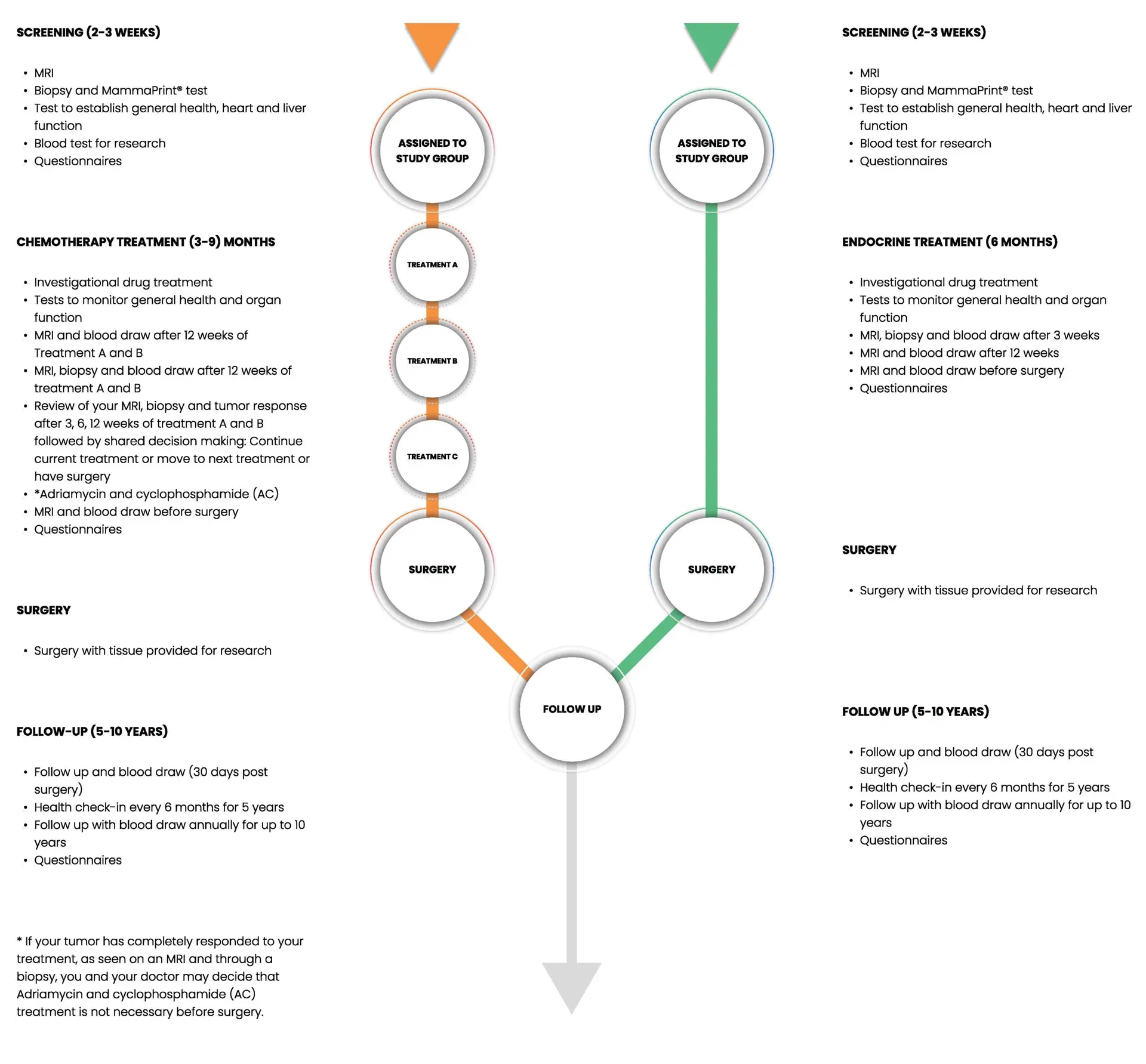
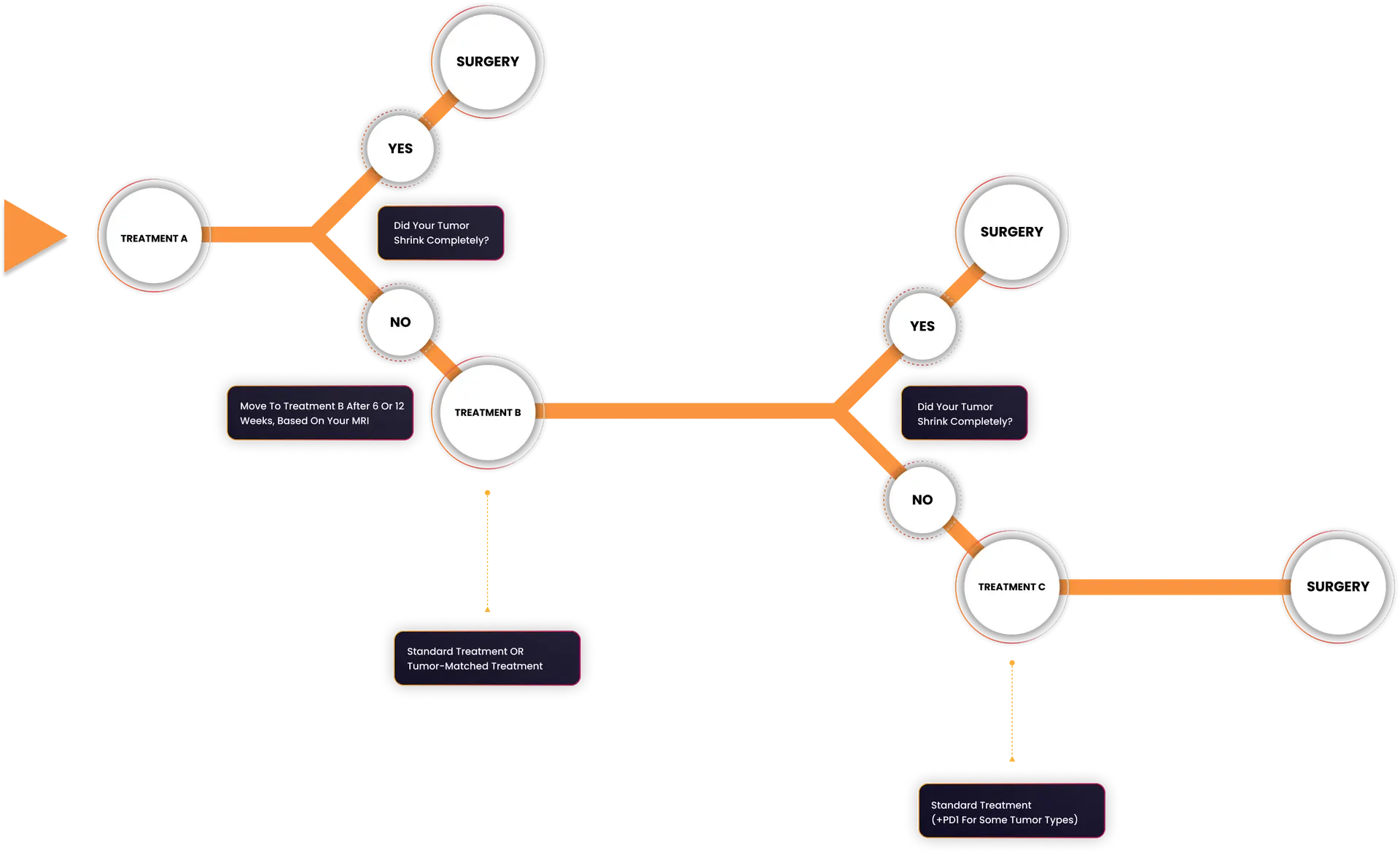
How the Treatment Phase Works
The diagram below shows how patients on the I-SPY 2 Chemotherapy Treatment Phase move through the trial. Patients are assigned a treatment based on their Response Predictive Subtype (RPS). They usually start with treatment A, which includes a novel targeted treatment for their tumor’s RPS. Patients may start with treatment B if there is not a suitable Treatment A option for their tumor type when they join the trial. If the treatment results in only a minimal response after the first treatment, they then move to Treatment B and if there is still tumor remaining, then to Treatment C. After any treatment, if the tumor has an excellent response and there is no evidence of cancer, there is the opportunity to proceed to surgery early, rather than continuing to take more treatment than is needed.
What drugs are used in the I-SPY 2 Trial?
Investigational Drugs
The investigational drugs in the I-SPY 2 trial are being tested to find drugs that are better than the standard treatment that is currently given to patients. We are also looking for drugs that are at least as good as the standard treatment but with fewer side effects. In addition, we are looking for biomarkers that could be used to select the most effective drug for each patient with the least side effects. In other words – the right drug for the right patient.
The investigational drugs available in the trial change over time. There are usually three or more drug treatments being tested but you and your doctor will not choose which one you get. A computer will randomly assign the drugs to you based on your specific tumor type. This is the most scientific way to reduce bias in a clinical trial. Information about your tumor and what we have learned from prior I-SPY 2 patients that have received each drug, will affect how the computer assigns drugs to you. This means that if an investigational drug is showing benefit to I-SPY 2 patients with tumors similar to yours, you will have an increased chance of receiving that drug.
Once you are assigned to a drug treatment, your study team will explain how the drug treatment works and what side effects you could experience. You can then choose to continue with the treatment phase of the trial or not.
Classes of investigational drugs
Information About Drugs Offered in the I-SPY 2 Trial
Different classes of investigational drugs work in the body in different ways.
One class of investigational drugs that we have tested is PARP inhibitors. PARP inhibitors prevent tumor cells from repairing damaged DNA. Cancer cells that cannot repair DNA damage are more likely to die. PARP inhibitors are effective when used with another chemotherapy drug, such as carboplatin, that causes DNA damage. It is most effective in cells that already have a defect in the DNA repair mechanism such as tumor cells from patients with BRCA mutations. Tumors that are identified by RPS as DNA repair deficient may respond well to PARP inhibitors.
Another class of investigational drugs being tested is called kinase inhibitors. Drugs belonging to this class interfere with the signaling mechanism that tells tumor cells to divide or die.
The I-SPY 2 trial is also testing immune-oncology investigational drugs such as PD-1/PD-L1 inhibitors. This class of investigational drug is showing promise in fighting many types of cancer. These block the cancer cell’s ability to protect itself, so the body’s immune system can destroy the cancer cells.
What drugs are used in the I-SPY 2 Trial?
Treatment B: Standard Chemotherapy Treatment
The I-SPY 2 trial uses the standard treatment as the comparison (control) group. If your breast cancer is HER2 negative the standard treatment is twelve weekly doses (referred to as cycles) of paclitaxel followed by four cycles of Adriamycin and cyclophosphamide (AC). An immune-oncology drug may be given with AC if your breast cancer is “triple negative”. If your breast cancer is HER2 positive the standard treatment includes either paclitaxel + trastuzumab + pertuzumab (THP), or Docetaxel + trastuzumab + pertuzumab + carboplatin (TCHP) . If you receive THP, you may also receive four cycles of Adriamycin and cyclophosphamide (AC). If you receive TCHP, you will have the option to have surgery after 4 cycles or 6 cycles, depending on your response to treatment.
Paclitaxel and Docetaxel are drugs that stops cancer cells from dividing and causes them to die. They do this by limiting the cells’ ability to divide in two.
Carboplatin, as well as Adriamycin and cyclophosphamide (AC) also stop cells from dividing and causes them to die, but they do this by tangling with the DNA to damage it. Using paclitaxel followed by Adriamycin and cyclophosphamide has been shown to be an effective treatment for many patients with breast cancer.
Trastuzumab and pertuzumab are HER2 targeted therapies that can slow or stop the growth of HER2 positive breast cancer.
(Additional information on common drugs mentioned can be found on the Cleveland clinic’s drug information site: Chemocare.com)
Information For I-SPY 2 Patients Who Are Eligible For Endocrine Treatment
For women with estrogen receptor positive breast cancer the standard treatment is anti-estrogen therapy, for example Tamoxifen or aromatase inhibitors (AIs). These work by either blocking estrogen from attaching to the breast cancer cells or by lowering the amount of estrogen in the body.
Patients participating in the Endocrine Treatment Phase will be randomly assigned to receive either a standard of care treatment with an aromatase inhibitors or one of many investigational agents for 6 months.
If you are a premenopausal or perimenopausal woman, you will also receive an oral ovarian suppression drug. This helps to prevent your body’s natural hormones from interfering with the study treatment.
Once you finish the treatment, you will have surgery to remove any tumor that may be left in your breast.
What should I do next?
As you are thinking about taking part in the I-SPY 2 Trial, you may find this trusted downloadable National Cancer Institute (NCI) booklet helpful, “Taking Part in Cancer Treatment Research Studies”.

If the I-SPY 2 Trial is of interest, you can use the I-SPY 2 Trial Patient Fact Sheet to discuss the trial with your doctor. You can also print or email it to family and friends to discuss your course of treatment and treatment decisions with them. Download or Print

















